Hydrogen atom neat labelled labeled spectral physics explain cbse toppr balmer brackett electrons
Table of Contents
Table of Contents
Are you struggling with drawing energy level diagrams? Don’t worry, you’re not alone. Many students find this topic to be challenging, but with the right guidance, you can achieve success.
When it comes to drawing energy level diagrams, students often struggle with determining the appropriate energy values, choosing the right orbitals, and understanding the concept of electron configuration. These pain points can make the process of drawing energy level diagrams frustrating and time-consuming.
At its core, drawing an energy level diagram involves understanding the arrangement of electrons in an atom or molecule. This arrangement happens in such a way that electrons occupy the lowest energy levels first, and the highest energy levels last. To draw an energy level diagram, you need to understand the energy values associated with each orbital and determine how many electrons can occupy each orbital.
To summarize, drawing an energy level diagram involves understanding the concept of electron configuration, determining energy values associated with each orbital, and knowing how many electrons can occupy each orbital.
How to Draw an Energy Level Diagram: A Personal Experience
When I first learned about energy level diagrams, I was overwhelmed and confused. However, with practice and guidance from my teacher, I was able to master this topic. One useful tip that helped me was to draw the energy level diagram step by step. For example, start with the lowest energy level and work your way up to the highest energy level.
Another helpful tip is to use diagrams or visual aids to help you understand electron configuration and orbital filling. This can make the process easier to understand and remember.
The Importance and Applications of Drawing Energy Level Diagrams
Energy level diagrams have a wide range of applications in chemistry and physics. They can be used to understand the electronic structure of atoms and molecules, determine the ionization potential of elements, and predict the reactivity of certain elements. Energy level diagrams are also critical in the study of spectroscopy, where they are used to identify the elements present in a sample.
Step-by-Step Guide to Drawing an Energy Level Diagram
To draw an energy level diagram, follow these steps:
- Determine the number of electrons in the atom or molecule you are drawing.
- Write the electron configuration of the element or molecule.
- Determine the energy values of each orbital.
- Fill the orbitals in ascending order of energy, following the Aufbau principle and Hund’s rule.
- Label the energy levels and orbitals in the diagram.
- Check your work to ensure that you have correctly filled all the orbitals and that the total number of electrons is correct.
Tips for Drawing Accurate Energy Level Diagrams
Here are some tips to help you draw accurate energy level diagrams:
- Pay close attention to the number of electrons in the atom or molecule.
- Use the electron configuration to determine the order of orbital filling.
- Follow the Aufbau principle and Hund’s rule.
- Label the energy levels and orbitals for easy reference.
- Practice, practice, practice!
Commonly Asked Questions
Q: What is the purpose of an energy level diagram?
A: An energy level diagram is used to show the arrangement of electrons in an atom or molecule. It helps chemists and physicists understand the electronic structure of elements and predict properties such as reactivity and ionization potential.
Q: What is the Aufbau principle?
A: The Aufbau principle states that electrons fill orbitals in order of increasing energy, starting with the lowest energy level first.
Q: What is Hund’s rule?
A: Hund’s rule states that for orbitals of equal energy, electrons enter singly with parallel spins before pairing up with opposite spins.
Q: How can I remember the order of orbital filling?
A: One useful method is to memorize the order: 1s, 2s, 2p, 3s, 3p, 4s, 3d, 4p, 5s, 4d, 5p, 6s, 4f, 5d, 6p, 7s, 5f, 6d, and 7p.
Conclusion of How to Draw an Energy Level Diagram
Drawing energy level diagrams can be challenging, but with a little practice and the right techniques, you can master this topic. Take the time to understand the principles of electron configuration, orbital filling, and energy values, and you’ll be well on your way to success.
Gallery
Draw Energy Level Diagram For Hydrogen Atom Showing At Least Four

Photo Credit by: bing.com / hydrogen atom emission regards transitions
How To Draw Energy Level Diagrams - YouTube

Photo Credit by: bing.com / energy level draw diagrams
How To Draw Energy Level Diagrams - YouTube
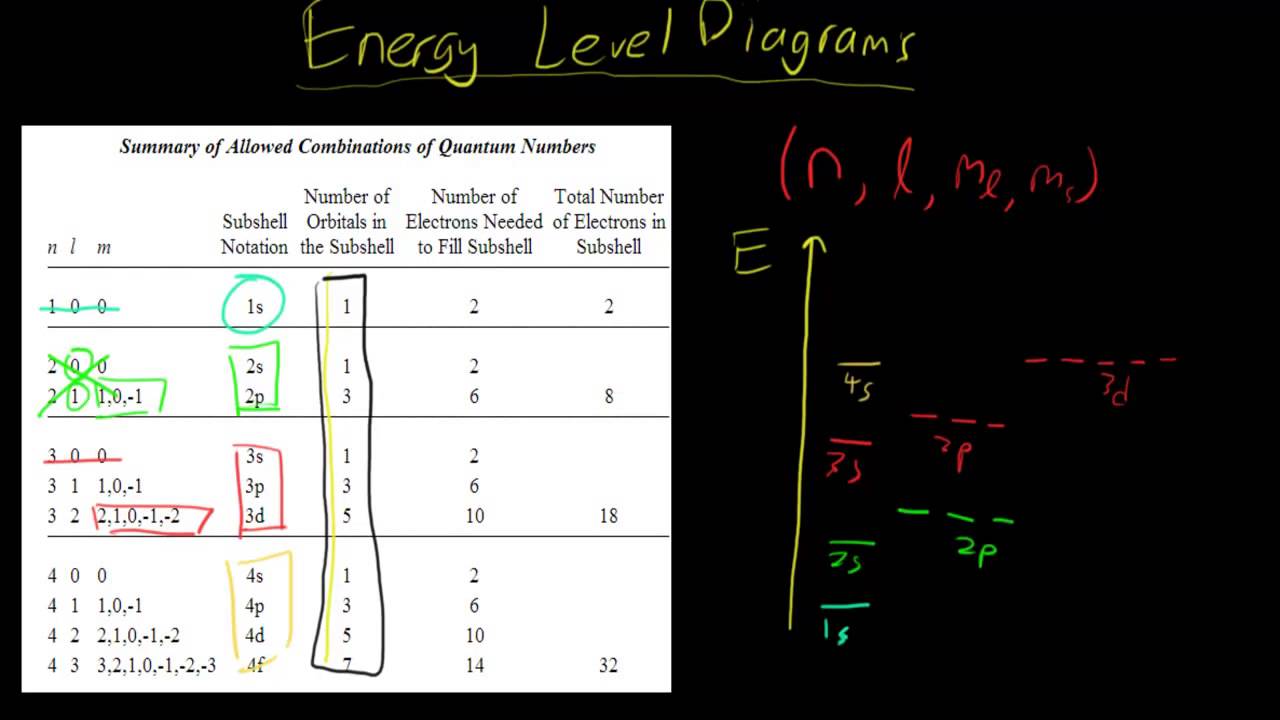
Photo Credit by: bing.com / energy level draw diagrams psx
Draw A Neat Labeled Energy Level Diagram And Explain Class 12 Physics CBSE

Photo Credit by: bing.com / hydrogen atom neat labelled labeled spectral physics explain cbse toppr balmer brackett electrons
13+ Partial Energy Level Diagram For Hydrogen - BessChristy
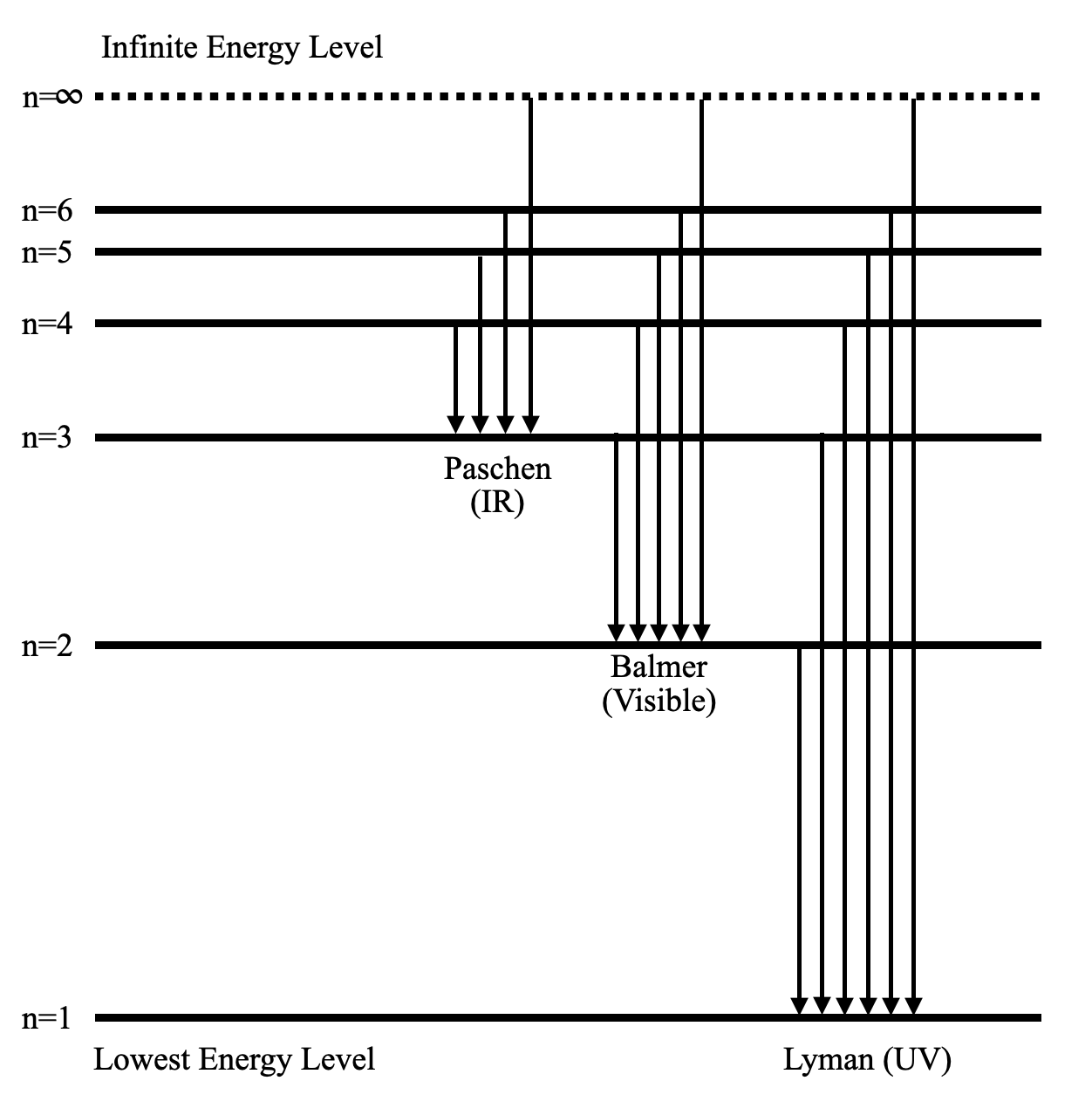
Photo Credit by: bing.com /